FACT SHEET - Rectal Microbicides
Fast Facts
- Rectal microbicides are products – that could take the form of gels, douches, suppositories or fast-dissolving rectal tablets called inserts – being developed and tested to reduce a person’s risk of HIV or other sexually transmitted infections from anal sex.
- The risk of becoming infected with HIV during condomless anal sex is 10 to 20 times greater than condomless vaginal sex. Because the rectal lining is only one-cell thick, the virus can more easily reach immune cells to infect.
- If proven effective, rectal microbicides could help protect against HIV in people who are unable or reluctant to use condoms every time they have sex, or who do not want to take a daily pill (pre-exposure prophylaxis, or PrEP) to reduce their risk of HIV. Just as there are multiple contraception options to prevent pregnancy, rectal microbicides could expand choices for HIV prevention by providing methods that are short-acting, non-systemic and used around the time of anal sex.
- Rectal microbicides are not yet available outside of clinical trials. Researchers first need to be sure they are safe and then conduct additional studies to find out whether they are effective against HIV.
- In the first Phase II trial of a rectal microbicide, completed in 2016, researchers found that a reduced glycerin formulation of tenofovir gel was safe and effective, particularly when used around the time of sex compared to daily use, among cisgender men and transgender women who engage in anal intercourse. Researchers are continuing to explore new potential products and delivery methods for rectal microbicides.
Overview
Microbicides are products designed to prevent or reduce the sexual transmission of HIV or other sexually transmitted infections when applied inside the vagina or rectum. Most vaginal microbicides are being tested as rings, while rectal microbicides are primarily being tested as gels. Microbicides currently under testing contain antiretroviral (ARV) drugs, many of which are commonly used to treat people with HIV. In 2012, the U.S, Food and Drug Administration (FDA) approved an ARV drug called Truvada as oral PrEP – a prevention approach in which HIV-negative people take an ARV tablet on a daily basis to reduce their risk of acquiring HIV.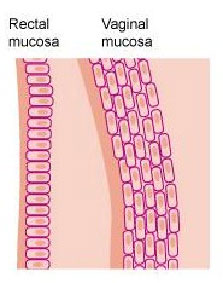
Although most microbicide research has focused on products to prevent HIV during vaginal sex, anal sex is practiced by people of all genders and sexualities around the world. According to some estimates, the risk of becoming infected with HIV through anal sex is 20 times greater than vaginal sex because the rectal lining, the mucosa, is thinner and much more fragile than the lining of the vagina.
An important first step in the development of rectal microbicides has been evaluating the rectal safety of microbicides originally formulated as vaginal gels. The most studied ARV-based vaginal microbicide has been tenofovir gel, which although found safe and moderately effective in reducing HIV risk in cisgender women who used it before and after vaginal sex in a study called CAPRISA 004, was found not effective in follow-up studies, VOICE and FACTS 001, due to low adherence to study products.
MTN researchers also have conducted studies of tenofovir gel as a rectal microbicide. Unlike the earlier studies, this research focused on individuals vulnerable to HIV from anal sex rather than vaginal sex. Because tenofovir gel could work differently against HIV in rectal tissue, researchers wanted to learn whether it was safe and acceptable to use rectally with an applicator. An early study found that the vaginal formulation of tenofovir gel caused gastrointestinal side effects when used in the rectum, so researchers tested a reformulated version of the gel with less glycerin in a follow-up study called MTN-007. That study found the reformulated gel to be safe and acceptable.
MTN has since completed a Phase II study of the reduced glycerin formulation of tenofovir gel among cisgender men and transgender women who engage in anal intercourse – the first ever of a rectal microbicide. The study, MTN-017, took place in Peru, South Africa, Thailand and the United States, including Puerto Rico. Results, announced in early 2016, found that the gel was safe, with participants preferring to use it around the time of sex compared to daily use.
Three additional MTN rectal microbicide studies have been completed and are anticipating results in 2020 – MTN-026, MTN-033 and MTN-037. MTN-026 and MTN-033 evaluated the rectal safety and pharmacokinetics of a gel based on dapivirine, an ARV that prevents HIV from replicating, while MTN-037 evaluated the safety of a multipurpose gel, PC-1005, developed by the Population Council to prevent HIV, herpes simplex virus (HSV) and human papillomavirus (HPV) simultaneously. HPV infection is common among people who engage in anal intercourse and is a primary risk factor for developing anal cancer, while HSV increases susceptibility to HIV. PC-1005 is the only product designed for vaginal and rectal use targeting all three sexually transmitted infections that has undergone a Phase 1 study to date. A rectal study currently underway, MTN-035, or DESIRE (Developing and Evaluating Short-acting Innovations for Rectal Use), is evaluating acceptability, tolerability and adherence to a placebo douche, suppository and rectal insert among 210 cisgender men and transgender men and women who engage in anal intercourse. DESIRE is the first study to systematically examine delivery methods for HIV prevention from receptive anal sex. The study is taking place in Malawi, Peru, South Africa, Thailand and the United States. Also underway is MTN-039, an important complement to DESIRE because it is evaluating a rectal insert that contains active products – tenofovir and elvitegravir.
Two Phase I studies, CHARM-01 and CHARM-02, comparing the safety, acceptability and distribution of three formulations of tenofovir gel – the vaginal formulation, the reduced glycerin formulation and a rectal-specific formulation – have been completed. These studies were led by the Combination HIV Antiretroviral Rectal Microbicide (CHARM) Program. An additional study, Project Gel, reported results in 2016, and found tenofovir gel safe and acceptable as a rectal microbicide in young cisgender men and transgender women who practice anal sex.
Other research-based programs underway include DREAM (Delivery of Rectal Enema as Microbicide), which is exploring the delivery of a rectal microbicide as a single dose enema (or douche), and PREVENT (Griffithsin-based Rectal Microbicides for Prevention of Viral Entry), which is addressing the need for a non-ARV based rectal microbicide.
Why Do We Need Rectal Microbicides?
Worldwide, nearly 38 million people are currently living with HIV. Although significant strides have been made in the treatment of HIV, now, more than 30 years after the HIV virus was first identified, the prevention of new infections continues to be great a challenge. An estimated 2 million new HIV infections occur annually – approximately 5,000 every day. HIV continues to disproportionately affect racial minorities and cisgender men and transgender women who have anal sex. In the United States, cisgender men who engage in anal sex account for 60 percent of all new HIV infections and represent more than half of the people currently living with HIV. Globally, this population is 19 times more likely to become HIV positive than the general population, with condomless anal sex as the primary reason.
According to estimates, 5 to 10 percent of the world’s population engages in anal sex. Condoms are an extremely effective method to prevent HIV during anal sex, but many people don’t use them correctly and consistently for a number of reasons – dynamics in sexual relationships, stigma about the practice of anal sex, or because they aren’t readily available in some countries. Similarly, PrEP – a strategy in which people take a pill called Truvada daily to prevent HIV– has been shown to be highly effective, but not everyone can or wants to take a pill every day. If proven safe and effective, rectal microbicides could provide an important additional prevention option – one that is short-acting, non-systemic and used around the time of sex.
What Will it Take to Discover a Safe and Effective Rectal Microbicide?
Drug development can take as many as 20 years before a single agent is approved for use. Thousands of potential compounds may be considered, but only the most promising products are subjected to rigorous laboratory and animal studies, and fewer still make it to trials with people. Clinical trials are carried out in several phases under the oversight of regulatory authorities and according to strict ethical and scientific guidelines. Phase I trials evaluate safety in a small number of people who are exposed to study products for short periods. If results suggest the product is safe, investigation progresses to a Phase II trial, in which researchers continue to track safety over longer periods of time. Phase IIb and III trials are performed to determine the effectiveness a product and conducted with large numbers of participants, often at multiple clinical centers. These trials usually compare a product with a placebo or another active product. Data from Phase IIb and III trials are often used by regulatory agencies to determine whether a particular product should be approved for widespread use.
Rectal microbicide research is in earlier phases of clinical development due in part to scientific challenges related to the biology of the rectum, and cultural reluctance to address anal sex. Several Phase I trials and one Phase II trial evaluating the rectal safety of microbicides have been completed to date. Many others are being developed, currently under way or expecting results.
Completed and Current Clinical Trials of Rectal Microbicides
- RMP-01 – A Phase I study of the rectal use of a gel containing UC781 that was found to be safe and well- tolerated in 36 cisgender men and women. Conducted by the University of California, Los Angeles (UCLA) in collaboration with the Division of AIDS-sponsored Integrated Preclinical/Clinical Program (IPCP) for HIV Topical Microbicides at National Institute of Allergy and Infectious Diseases.
- RMP-02/MTN-006 – A Phase I study of tenofovir gel applied rectally compared to oral tenofovir. Compared to a placebo gel, the gel significantly inhibited HIV in rectal tissue taken from 18 cisgender men and women in the U.S. who used it daily for one week. To address side effects in a few participants, researchers subsequently reformulated the gel. Conducted by the MTN and UCLA/IPCP.
- MTN-007 – A Phase I follow-up study to RMP-02/MTN-006 testing the rectal use of a reformulated version of tenofovir gel that was found safe and acceptable in 65 cisgender men and women in the U.S. Conducted by the MTN.
- MTN-017 – A Phase II rectal safety and adherence study of reformulated version of tenofovir gel used daily and before and after sex, and oral Truvada. The study, which enrolled 195 cisgender men and transgender women who engage in anal intercourse, found the gel was safe and most acceptable to participants when used before and after sex compared to daily use. The study was conducted by the MTN at sites in Peru, South Africa, Thailand and the U.S., including Puerto Rico.
- MTN-026 – A Phase I safety and acceptability study of dapivirine formulated as a rectal gel, launched in late 2017 and awaiting results. Conducted by MTN.
- MTN-033 – A Phase I study of the safety and distribution of dapivirine gel when administered with an applicator compared to a phallic device, launched in mid-2018 and awaiting results. Conducted by MTN.
- MTN-035 – A study assessing acceptability, tolerability and adherence to placebo rectal microbicides in three formulations: douche, suppository and rectal insert. Launched in early 2019, and taking place at sites in Malawi, Peru, South Africa, Thailand and the U.S. Conducted by MTN.
- MTN-037 – A Phase 1 safety and pharmacokinetic study of a rectal gel called PC-1005 developed by the Population Council, launched in June 2018 and awaiting results. Conducted by MTN.
- MTN-039 – A Phase 1 safety and pharmacokinetic study of a tenofovir and elvitegravir rectal insert launched in late 2019. Product developed by CONRAD and study conducted by MTN.
- CHARM-01 & CHARM-02 – Phase I studies that compared the safety, acceptability and distribution of three formulations of tenofovir gel – a vaginal formulation, a reduced glycerin formulation and a rectal-specific formulation, and found all three safe and acceptable. Conducted by the CHARM Program.
- Project Gel – Multi-stage trial focused on rectal microbicide acceptability, safety and adherence in young cisgender men and transgender women who engage in anal intercourse at sites in the U.S., including Puerto Rico, that found tenofovir gel safe and acceptable. Conducted by the University of Pittsburgh.
# # #
The Microbicide Trials Network (MTN) is an HIV/AIDS clinical trials network established in 2006 by the National Institute of Allergy and Infectious Diseases with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. Based at Magee-Womens Research Institute and the University of Pittsburgh, the MTN brings together international investigators and community and industry partners whose work is focused on the development and rigorous evaluation of promising microbicides – products applied inside the vagina or rectum that are intended to prevent the sexual transmission of HIV – from the earliest phases of clinical study to large-scale trials that support potential licensure of these products for widespread use. More information about the MTN is available at http://www.mtnstopshiv.org.
Click here for PDF version of this document.
21-April-2020
