The DELIVER (MTN-042) and B-PROTECTED (MTN-043) Studies:
Meeting the HIV Prevention Needs of Pregnant and Breastfeeding Women
Key Points and Fast Facts
- Women need safe and effective methods for HIV prevention they can use at all times of their lives, including, if not especially, during pregnancy and breastfeeding, when they are up to four-times more likely to acquire HIV.
- Daily use of an antiretroviral (ARV) pill called Truvada®, an approach known as PrEP (short for pre-exposure prophylaxis) and the monthly dapivirine vaginal ring, a new HIV prevention method approved in several African countries and recommended by the World Health Organization (WHO), were found to be well tolerated and reduce the risk of HIV in clinical trials in cisgender women, who were not pregnant or breastfeeding.
- Most of what is known about the safety of Truvada (which contains emtricitabine and tenofovir disoproxil fumarate) during pregnancy and breastfeeding is based on its use for the treatment of HIV in combination with other drugs, with a growing body of evidence finding it safe to use for HIV prevention during pregnancy and breastfeeding as well. Much less is known about the safety of the dapivirine ring during pregnancy and breastfeeding.
- DELIVER (MTN-042) and B-PROTECTED (MTN-043) are Phase IIIb studies that were designed to evaluate the safety and acceptability of the dapivirine ring during pregnancy and breastfeeding, respectively, as well as collect additional safety data about Truvada as daily PrEP in pregnant and breastfeeding populations.
- Both studies aim to provide the kind of data regulatory authorities and national programs need to consider making the dapivirine ring available to pregnant and breastfeeding women, as well as help health care providers, and women themselves, make informed decisions about whether to use the ring (or PrEP) while pregnant or breastfeeding.
- Results of B-PROTECTED, which were presented at the 2023 Conference on Retroviruses and Opportunistic Infections (CROI 2023), suggests the dapivirine ring is safe to use during breastfeeding. Although DELIVER is still ongoing, results to date, also reported at CROI 2023, found no safety concerns with use of the ring during the third trimester of pregnancy. DELIVER is expected to be completed in 2023 with final results in women who started using the ring or PrEP earlier in pregnancy anticipated late 2024 or early 2025.
DELIVER and B-PROTECTED: Overview, Context and Need
Who is conducting and funding the DELIVER and B-PROTECTED studies?
Both DELIVER (MTN-042) and B-PROTECTED (MTN-043) are studies that were designed and implemented by the Microbicide Trials Network (MTN), which from 2006 until November 30, 2021, was funded as an HIV/AIDS Clinical Trials Network by the National Institute of Allergy and Infectious Diseases (NIAID), Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute of Mental Health, all components of the U.S. National Institutes of Health. MTN’s portfolio of studies included among the first HIV prevention studies involving pregnant and breastfeeding women, a research agenda that is continuing with NIAID’s support until the completion of DELIVER.
What is the current status of these studies?
B-PROTECTED, which enrolled 197 HIV-negative cisgender breastfeeding women and their 6- to 12-week-old babies, was conducted between September 2020 and November 2021. Results of B-PROTECTED, which were presented at the 2023 Conference on Retroviruses and Opportunistic Infections (CROI 2023), found use of the dapivirine ring during breastfeeding posed no safety concerns.
DELIVER, which began in February 2020, has completed its first two cohorts of participants, with 150 women in Cohort 1 who were 36-plus weeks gestation (8-9 months pregnant) when they enrolled and 157 participants in Cohort 2 who were 30- to 35-week gestation (7-8 months pregnant) at the time they joined the study. Follow-up of women in the third and final cohort – 251 participants who were 12-29 weeks gestation (3-7 months pregnant) at enrollment – is expected to be completed by mid-2023. Because babies are followed for an additional year after birth, final results are anticipated late 2024 or early 2025.
Where was B-PROTECTED conducted and is DELIVER taking place?
The same NIAID-funded clinical research sites (CRSs) that conducted B-PROTECTED are also conducting DELIVER. These are: the College of Medicine-Johns Hopkins University Research Project in Blantyre, Malawi; Wits Reproductive Health and HIV Institute (Wits RHI) Shandukani Research Centre in Johannesburg, South Africa; Makerere University–Johns Hopkins University (MU-JHU) Research Collaboration in Kampala, Uganda; and the University of Zimbabwe Clinical Trials Research Centre Zengeza (UZ-CTRC) CRS in Harare.
Who is leading these studies?
B-PROTECTED was led by Maxensia Owor, MBChB, MMed (Paed), MPH, from MU-JHU in Kampala, Uganda, who was the study’s protocol chair; with Lisa Noguchi, PhD, CNM, Johns Hopkins Bloomberg School of Public Health, Baltimore; and Jennifer Balkus, PhD, MPH, from the University of Washington School of Public Health in Seattle, serving as protocol co-chairs. DELIVER is being led by protocol chairs Katherine Bunge, MD, MPH, of the University of Pittsburgh School of Medicine, and Felix G. Mhlanga, MBChB, MMed, from UZ-CTRC; and Lee Fairlie, MBChB, FCPeds (SA), MMED (Paeds), from Wits RHI in Johannesburg, South Africa, who is the protocol co-chair.
Why is it important to know about a drug’s safety during pregnancy and breastfeeding? What are the potential concerns?
Understanding a drug’s safety during pregnancy and breastfeeding helps women and their healthcare providers make good decisions about medication use. A woman’s body undergoes many changes during pregnancy and breastfeeding that could affect how a drug gets absorbed and distributed – a drug may not work as well or its use may not be safe, causing harm to the mother, her pregnancy or baby. While most drugs get passed into breastmilk at low levels, it’s important to know how much and at what levels a particular drug might pose a risk for the baby.
What was known about the safety of Truvada as PrEP in pregnant and breastfeeding women – before DELIVER and B-PROTECTED?
Truvada as PrEP is approved in many countries, and, in several settings, being offered to pregnant and breastfeeding women. Most of the information about Truvada’s safety during pregnancy and breastfeeding is from its use as HIV treatment in combination with other drugs, with a growing body of evidence suggesting it is safe to use as HIV prevention during pregnancy and breastfeeding as well. Because lower drug levels have been noted during pregnancy among women living with HIV, researchers conducting the IMPAACT 2009 study sought to learn whether the same would be true in pregnant HIV-negative adolescent girls and young women ages 16-24 using Truvada as PrEP. Indeed, results of the first phase of the study, which were reported early 2020, found drug levels were 31 to 37 percent lower during pregnancy than in the postpartum period, indicating that full adherence to daily pill taking is especially important during pregnancy. IMPAACT 2009, which is being conducted at the same sites as DELIVER and B-PROTECTED, will provide additional information about the safety of PrEP during pregnancy and breastfeeding as well.
What was known about the safety of the dapivirine ring in pregnant and breastfeeding women – before DELIVER and B-PROTECTED?
Though animal studies of dapivirine indicate no concerns related to pregnancy or fetal development, before DELIVER, the only human data was from about 240 women who became pregnant while using the dapivirine ring during the Phase III trials (ASPIRE/MTN-020 and The Ring Study/IPM-027) and stopped using the ring outcomes between women assigned to use the dapivirine ring and those assigned to use a placebo who became pregnant, suggesting ring use during conception and early pregnancy is not harmful. DELIVER will provide information about the ring when it is used for longer periods and at different stages during pregnancy.
The only information about the possible safety of the dapivirine ring during breastfeeding was from MTN-029/IPM 039 involving 16 participants in the US who had already weaned their babies but were still producing milk and who used the ring for 14 days. The study was designed so that babies would not be exposed to drug, but seeing that very low levels of drug were detected in maternal breastmilk, researchers estimated that an infant’s daily exposure to drug would likely be very low – information that would need to be confirmed in a study of women who are actively breastfeeding and using the ring.
DELIVER and B-PROTECTED: What we have learned so far
How was the B-PROTECTED study designed?
B-PROTECTED was designed to enroll approximately 200 HIV-negative cisgender breastfeeding women and their 6- to 12-week-old babies with women being randomly assigned to use either the monthly dapivirine ring or Truvada as daily oral PrEP for three months. Because more information is needed about the safety of the dapivirine ring, three times as many participants assigned to use ring than PrEP. Both mothers and their babies were followed for an additional two weeks after they stopped using study product
At different time points during the study, researchers assessed how much drug from each product could be measured in blood samples from the mothers, how much was present in breast milk, and how much could be measured in blood samples from the babies. At each study visit, clinic staff also conducted interviews and physical exams to check for any new signs or symptoms in mothers and their infants and determine their possible cause, i.e., whether or not they may be due to use of either the ring or oral PrEP.
How many participants did B-PROTECTED enroll?
B-PROTECTED enrolled 197 participants, 148 of whom were assigned to use the dapivirine ring (inserting a new ring each month) and 49 women assigned to use Truvada as daily oral PrEP.
What did B-PROTECTED find?
Results of B-PROTECTED, which were presented at CROI 2023, suggest the dapivirine ring can be safely used during breastfeeding. While drug could be detected in breastmilk, the absolute levels were very low and the amount of drug ingested by babies even lower, posing no safety concerns. Dapivirine levels in breastmilk were similarly low to what was found in the MTN-029/IPM-039 study, and the very small amount of drug infants were actually exposed to via breast milk was very small. As expected, results also found drug levels for Truvada (as measured by tenofovir diphosphate) were low as well, confirming what has been seen in other studies.
How was the DELIVER study designed?
DELIVER was designed to enroll approximately 550 HIV-negative women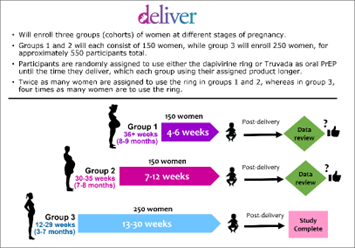 between the ages of 18 and 40 and to be conducted in stepwise fashion, enrolling one group of women at a time, beginning with women late in pregnancy, and participants being randomly assigned to use either the dapivirine ring or Truvada as daily PrEP until the time they deliver. Moreover, the study would only proceed to the next cohort of participants unless an independent panel of experts deemed it safe to do so based on its review of data from the previous cohort. In Cohorts 1 and 2, twice as many participants were to be assigned to use the dapivirine ring than Truvada as oral PrEP, and in the third and final cohort, four times as many participants would use the ring.
between the ages of 18 and 40 and to be conducted in stepwise fashion, enrolling one group of women at a time, beginning with women late in pregnancy, and participants being randomly assigned to use either the dapivirine ring or Truvada as daily PrEP until the time they deliver. Moreover, the study would only proceed to the next cohort of participants unless an independent panel of experts deemed it safe to do so based on its review of data from the previous cohort. In Cohorts 1 and 2, twice as many participants were to be assigned to use the dapivirine ring than Truvada as oral PrEP, and in the third and final cohort, four times as many participants would use the ring.
While in the study, participants are seen by clinic staff every two to four weeks and asked about their health and experience using the ring or PrEP and whether they are having any problems, such as headache, nausea, pain or discomfort. In addition, women undergo physical exams, and laboratory tests are conducted. Women are to be followed for an additional six weeks after giving birth, and their babies are in the study for one year.
How many participants were enrolled in each Cohort?
In total, DELIVER has enrolled 558 participants. Cohort 1 enrolled 150 women who were 36-plus weeks (8-9 months) pregnant, of whom 101 were randomly assigned to use the dapivirine ring and 49 to use Truvada as oral PrEP. Of the 157 participants in Cohort 2, who were between 30- and 35-weeks gestation (7-8 months pregnant) when they joined the study, 106 used the ring and 51 used oral PrEP. The third and final cohort has enrolled 251 women who were between 12- and 29-weeks gestation (3-7 months pregnant) at the time they joined the study, with approximately200 participants who were assigned to use the dapivirine ring.
How is the safety of the dapivirine ring and Truvada as PrEP being assessed in DELIVER?
Though most pregnancies are “uneventful,” pregnancy is not without risks, and, as such, it is expected there will be participants in DELIVER who experience complications, some which may be serious. As part of the study, the research team documents all pregnancy outcomes (whether it was a full-term live birth, premature birth or stillborn), the method of delivery (vaginal or Cesarean) and the infant’s birth weight. The team also assesses the health of both babies and mothers, keeping track of certain complications. These include complications associated with high blood pressure, or so-called hypertensive disorders of pregnancy (gestational hypertension, eclampsia and preeclampsia); postpartum endometritis, an infection in the uterus that develops after childbirth; chorioamnionitis, an infection in the uterus affecting the amniotic sac or its membranes; and postpartum hemorrhage, or excessive bleeding after childbirth.
With no placebo group in DELIVER, researchers needed a frame of reference to determine whether the particular complications or adverse events observed among women in the study are occurring with similar frequency to what can be expected for women locally. Data collected through the MTN-042B sub-study, which involved a review of more than 10,000 medical records at the same hospitals and clinics where DELIVER study participants would give birth, provided researchers with the basis for comparison needed to evaluate the safety of PrEP and the dapivirine ring during pregnancy. This same data was used as a frame of reference for the independent panel of experts who after each cohort determined whether based on its review of study data the study should continue with enrollment of the next group.
What have we learned from DELIVER so far?
DELIVER has already found use of the dapivirine ring during the third trimester of pregnancy posed no safety concerns, with pregnancy outcomes and complications experienced by participants in the first and second cohorts no different than what would be expected of women in the local communities where DELIVER is being conducted.
Reporting at CROI 2023, researchers said the vast majority of participants in both cohorts delivered full-term (between 37 and 42 weeks) live births, with only 2 percent of births delivered prematurely across both groups of women in Cohort 1 (those who used the dapivirine ring and those who used oral PrEP), and in Cohort 2, 6 percent of the births being premature, lower than the 13 percent premature birth rate expected within the trial site communities (though Cohort 1 were almost full term to begin with). There were two stillbirths, one in each cohort, neither of which was deemed related to use of the study product. In cohort 1, the stillbirth occurred in a participant assigned to Truvada as oral PrEP, whereas in Cohort 2 the stillbirth was experienced by a participant using the dapivirine ring. While based on data collected through the MTN-042B sub-study, 4 percent of pregnancies could be expected to result in a stillbirth, the frequency for each product and each cohort was well below this background rate. For Cohort 1, the frequency of stillbirth in the oral PrEP group was 2 percent and 0.7 percent for the cohort as a whole; and for Cohort 2, the frequency of stillbirth was 1 percent for the dapivirine ring and 0.6 percent for the Cohort as a whole.
Complications associated with high blood pressure (hypertensive disorders of pregnancy) were the most common complications experienced by study participants, but at rates lower than or similar to local background rates of 10.5 percent based on the records review. Hypertensive disorders of pregnancy were experienced by 3 percent of dapivirine ring users in Cohort 1 and 8 percent for Cohort 2; while for oral PrEP, these occurred in 8 percent of Cohort 1 and 10 percent in Cohort 2.
Have any participants in either study acquired HIV? What would happen if this should be the case? And what will be done to protect her baby?
No participants in the B-PROTECTED study acquired HIV, and no HIV infections were seen in the first two cohorts of women in DELIVER. Cohort 3 of DELIVER is ongoing, and these participants, like those in the previous two groups and participants in B-PROTECTED, are instructed to use their assigned study product consistently – daily for PrEP, and for the ring, to leave it in for a full month at a time. Participants also receive free condoms, frequent HIV testing and HIV risk-reduction counseling, and routine testing and treatment for sexually transmitted infections. Despite these protective measures, a participant could still acquire HIV. If a participant tests positive for HIV, she will be linked immediately to services providing treatment and to prevent transmission of HIV to her baby. Participants stop using the study products, but they and their baby can still come for study visits.
What kind of approvals were required to conduct these studies?
Both the DELIVER and B-PROTECTED studies underwent extensive and rigorous review by NIAID and the US Food and Drug Administration (FDA). Moreover, before any site could begin enrolling women into either study, approvals were required of national regulatory authorities in the trial site country and by the site’s Institutional Review Board (IRB) or Ethics Committee (EC). IRBs and ECs ensure studies are scientifically valid and ethically sound and provide oversight throughout the duration of the trial.
What are community views about the use of PrEP and the ring during pregnancy and breastfeeding?
To understand community attitudes and perceptions about the use the ring and PrEP during pregnancy and breastfeeding, researchers conducted the MTN-041 (MAMMA) study in the same communities in Malawi, South Africa, Uganda and Zimbabwe where DELIVER and B-PROTECTED studies would take place. MTN-041 involved group discussions with pregnant and breastfeeding women, male partners, mothers and mothers-in-law, and interviews with community leaders, health care providers, midwives and traditional birth attendants, among others. Across all groups, there was an appreciation that women were at high risk of HV during pregnancy and breastfeeding and a need for different methods of protection such as the vaginal ring and oral PrEP. For these products to be accepted, many felt buy-in of healthcare providers would be key. The influence that male partners have over women’s decision making and behavior was also emphasized, though in some settings, mothers and mothers-in-laws were deemed equally, if not more, influential.
More about the dapivirine ring and oral PrEP
What is the dapivirine vaginal ring?
The monthly dapivirine vaginal ring is the first biomedical HIV prevention product designed specifically for women. The ring is made of a flexible silicone material containing 25mg of the ARV drug dapivirine, about 4 mg of which is released into the vagina when used continuously for 28 days with low absorption elsewhere in the body. Women can insert and replace the ring themselves each month.
The dapivirine ring, which was developed by the non-profit International Partnership for Microbicides (IPM), received a positive scientific opinion from the European Medicines Agency in July 2020 for its use by cisgender women at high risk for HIV who cannot or choose not to use daily oral PrEP, and in 2021, WHO recommended the ring as an additional prevention option for women. Countries that have approved the dapivirine ring include Kenya, Rwanda, South Africa, Uganda and Zimbabwe, among others. In mid-2022, the dapivirine vaginal ring and other IPM assets were transferred to the Population Council, a global nonprofit research organization.
What is oral PrEP?
PrEP, which stands for pre-exposure prophylaxis, is an HIV prevention method in which people who don’t have HIV take an ARV pill daily to reduce their risk of being infected. WHO recommends oral PrEP for anyone at significant HIV risk. The ARV pill most commonly used as PrEP is Truvada, the brand name for a tablet containing the ARVs tenofovir disoproxil fumarate and emtricitabine that is marketed by Gilead Sciences of Foster City, California. Truvada was originally developed (and still used) for the treatment of HIV, in combination with other ARVs. Truvada was first approved for use as PrEP by the US FDA in 2012 and has since been approved in more than 50 countries.
In 2019, Gilead obtained FDA approval of a second drug for PrEP called Descovy®, which contains emtricitabine and tenofovir alafenamide (F/TAF). Approval does not apply to people at risk of getting HIV through receptive vaginal sex, because effectiveness in this population has not yet been evaluated. The safety and efficacy of F/TAF among cisgender adolescent girls and women is being evaluated as part of a trial Gilead is conducting in South Africa and Uganda.
# # #
The MTN was supported by U.S. National Institutes of Health grants UM1AI068633, UM1AI068615 and UM1AI106707.
More information about the MTN is available at www.mtnstopshiv.org. To learn more about the DELIVER (MTN-042) and B-PROTECTED (MTN-043) go to https://mtnstopshiv.org/news/studies/mtn042 and https://mtnstopshiv.org/news/studies/mtn043.
Click here for PDF version of this document.
21-February-2023
